How To Solve Solution Concentration Problems
Calculate the initial millimoles of the acid and the base. The percent by mass would be calculated by.

5 Easy Ways To Calculate The Concentration Of A Solution
The thin-film solution The thin-film solution can be obtained from the previous example by looking at the case where Δx is very small compared to the diffusion distance x and the thin film is initially located at x 0.
How to solve solution concentration problems. Determine H and convert this value to pH. A solution is prepared by dissolving 364 g CaI2 in 750 mL of water. Concentration is the amount of solute salt in this case divided by the volume of the solution brine in this case.
The following video looks at calculating concentration of solutions. The concentration and the volumes change in a dilution. We will look at another Sample problem dealing with volumevolume percent vv.
Cxt N 4Dt ex 24Dt where N is the number of. Explain what changes and what stays the same when 100 L of a solution of NaCl is diluted to 180 L. It is the amount of solute dissolves in 100 g solvent.
Suppose that a solution was prepared by dissolving 250 g of sugar into 100 g of water. Solution Concentration Problems A solution is prepared by dissolving 267 g of NaOH in 650. Concentration is an expression of how much solute is dissolved in a solvent in a chemical solutionThere are multiple units of concentrationWhich unit you use depends on how you intend to use the chemical solution.
Convert the proportion to a decimal by dividing the numerator by thedenominator. Mixture problems involve combining two or more things and determining some characteristic of either the ingredients or the resulting mixture. The number of moles always stays the same in a dilution.
What is the molality of the solution. For example we might want to know how much water to add to dilute a saline solution or we might want to determine the percentage of concentrate in a jug of orange juice. What is the mole fraction of the sodium hydroxide.
If concentration of solution is 20 we understand that there are 20 g solute in 100 g solution. The following video looks at calculating concentration of solutions. We can use fractions ratios or percentages to describe.
Set up a proportion with the amount of active ingredient listed over thetotal quantity as grams over grams. We will look at another Sample problem dealing with massvolume percent mv. Find concentration of solution by percent mass.
Calculate the concentrations of all the species in the final solution. Multiply the converted number by 100 to express the finalconcentration as a percentage. Use a tabular format to determine the amounts of all the species in solution.
So assuming that the unit of x the amount of salt is pounds the concentration of the brine in the tank at the start is x 100 lb gal However the volume in the tank does not remain at 100 gallons. The most common units are molarity molality normality mass percent volume percent and mole fraction. When the solute in a solution is a solid a convenient way to express the concentration is a mass percent which is the grams of solute per 100 g of solution.
10 g salt and 70 g water are mixed and solution is prepared. Henrys law shows that the concentration of a solute gas in a solution is directly proportional to the partial pressure of the gas over the solutionP KHC whereP is the partial pressure of the gas above the solutionKH is the Henrys law constant for the solutionC is the concentration of the dissolved gas in solutionC PKHC 24 atm29.

Solving Systems Of Equations Real World Problems Word Problems Word Problem Worksheets Graphing Linear Equations
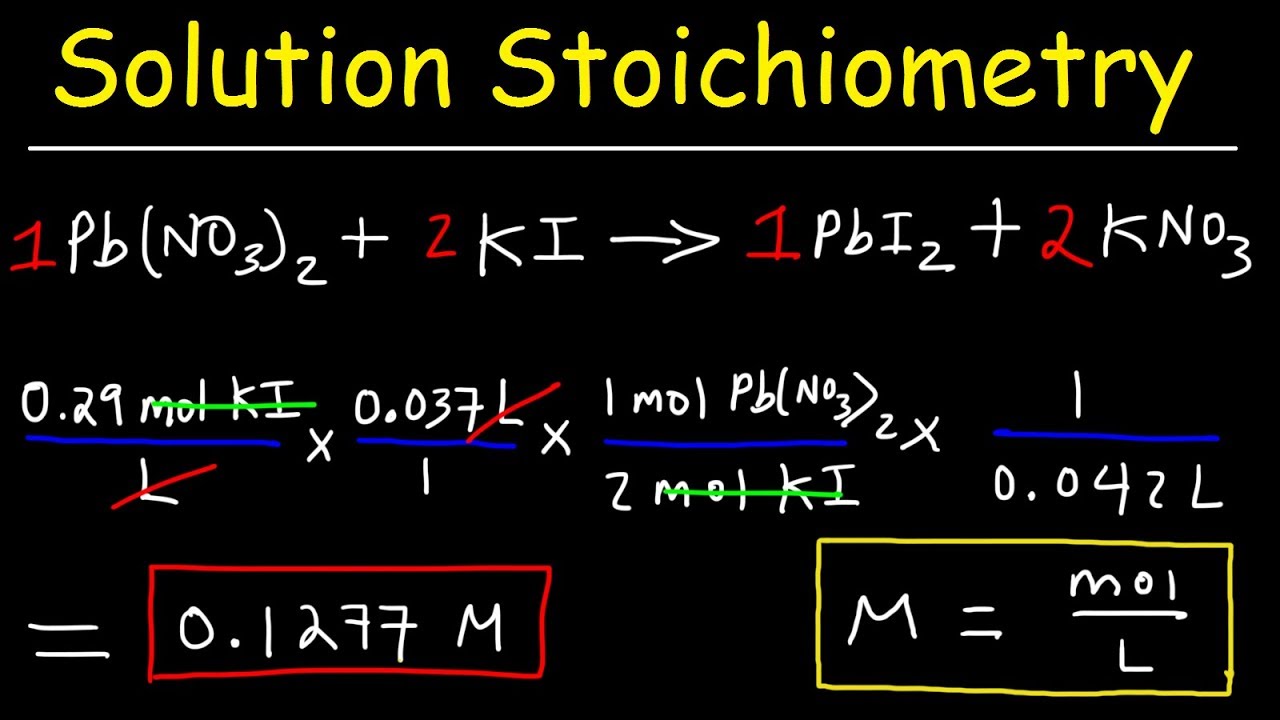
Dilution Problems Chemistry Molarity Concentration Examples Formula Equations Youtube

Are You Smart Enough To Solve This Tricky Math Problem Order Of Operations Math Solving
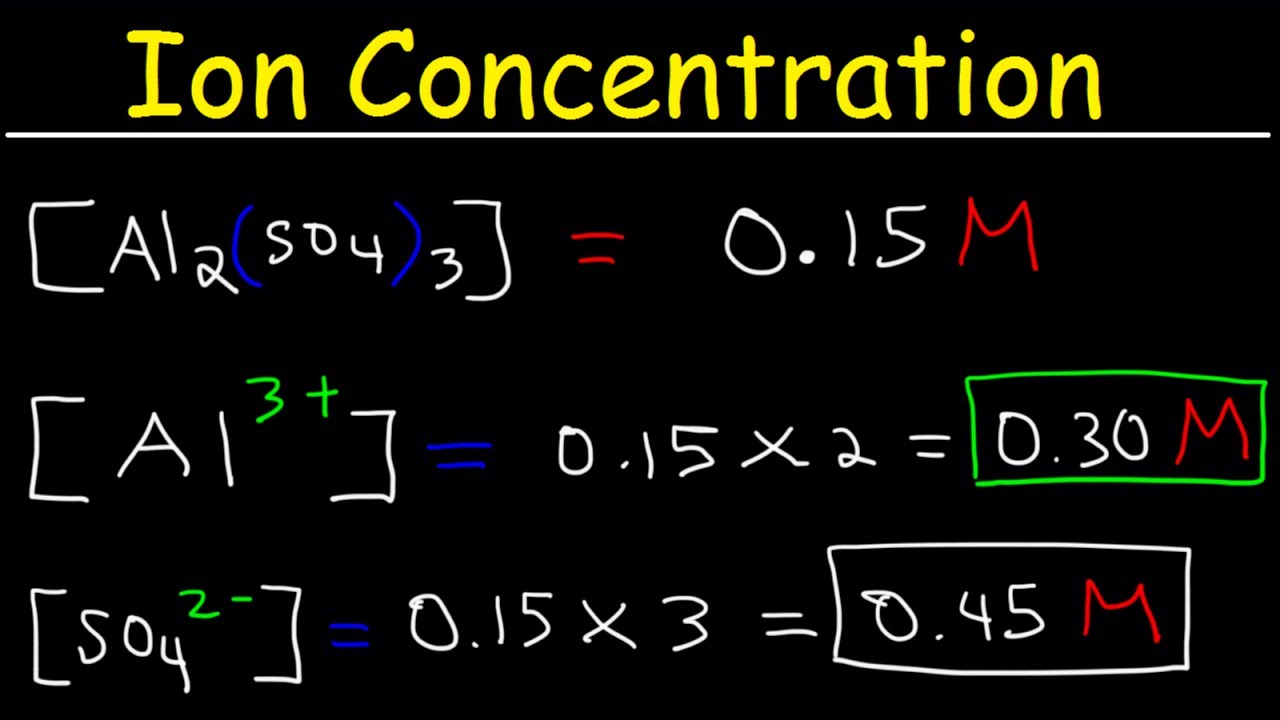
Molarity Practice Problems Youtube

Equilibrium Expressions Using Keq To Solve Problems Chemistry Worksheets Problem Solving Worksheet Solving Word Problems

Dilution Problems Chemistry Molarity Concentration Examples Formula Equations Youtube

Mass Percent Of A Solution Made Easy How To Calculate Mass Or Make A Solutions Mass Make It Simple

Buffer Solution Ph Calculations Henderson Hasselbalch Equation Explained Chemistry Problems Youtube Buffer Solution Chemistry Science Chemistry

5 Easy Ways To Calculate The Concentration Of A Solution

Speed Math Puzzle Distance Rate Time Word Problems Word Problems Time Word Problems Sped Math

Solution Concentration Boundless Chemistry

5 Easy Ways To Calculate The Concentration Of A Solution

Solution Concentration Boundless Chemistry

5 Easy Ways To Calculate The Concentration Of A Solution

Applications Of First Order Differential Equations Mixing Concentrations Differential Equations Equations Concentration

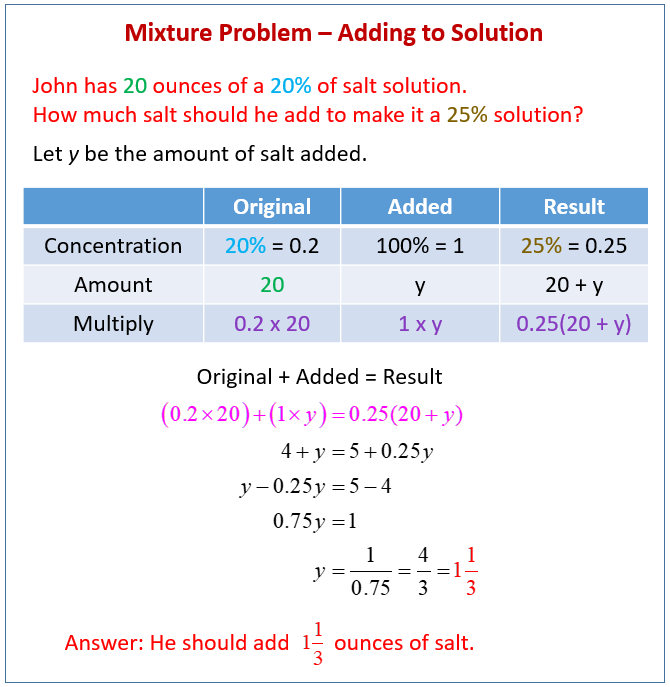
Mixture Word Problems Video Lessons Examples And Solutions

What Is Diophantine Equation Examples With Solutions Diophantine Equation Quadratics Algebra Equations

5 Easy Ways To Calculate The Concentration Of A Solution
